TL;DR
Clinical Evidence in Digital Health: Proven clinical efficacy and safety, demonstrated through trials and regulatory approvals, are critical for digital health solutions to gain acceptance from healthcare stakeholders.
Partnerships Fuel Growth: Collaborations with pharmaceutical companies and healthcare providers are essential for digital health ventures, providing access to resources and credibility.
Key Areas Requiring Validation: Sectors like diagnostic tools, remote monitoring devices, and patient apps are under greater scrutiny, as they need to demonstrate higher clinical strength due to their direct impact on patient care and safety.
Investment Trends: Investors are increasingly funding digital health ventures that have strong clinical evidence, as they present lower risk and a higher chance of successful market adoption.
In the rapidly evolving world of digital health, where innovation drives the future of healthcare, establishing clinical evidence in digital health is more crucial than ever. As the sector matures, the need for rigorous scientific validation has emerged as a non-negotiable element in gaining the trust of healthcare providers, patients, payers, and investors alike. With the full report on clinical strength in digital health set to be released at the end of September, this blog serves as a teaser, highlighting key insights and inviting you to subscribe to be notified when the report becomes available.
How to build Clinical Evidence for Digital Health?
To establish clinical strength in digital health, ventures must rigorously demonstrate their solutions’ efficacy and safety. This involves conducting clinical trials, obtaining regulatory approvals, and publishing research in peer-reviewed journals. Going beyond mere technological innovation, clinical strength ensures that digital health solutions deliver tangible health benefits in controlled settings.
In today’s competitive landscape, clinical strength is not optional but essential. Whether regulated or not, clinically validated solutions are more likely to be adopted by healthcare providers and investors. Corporate partners and investors increasingly demand solid evidence of clinical efficacy before committing to partnerships or funding.
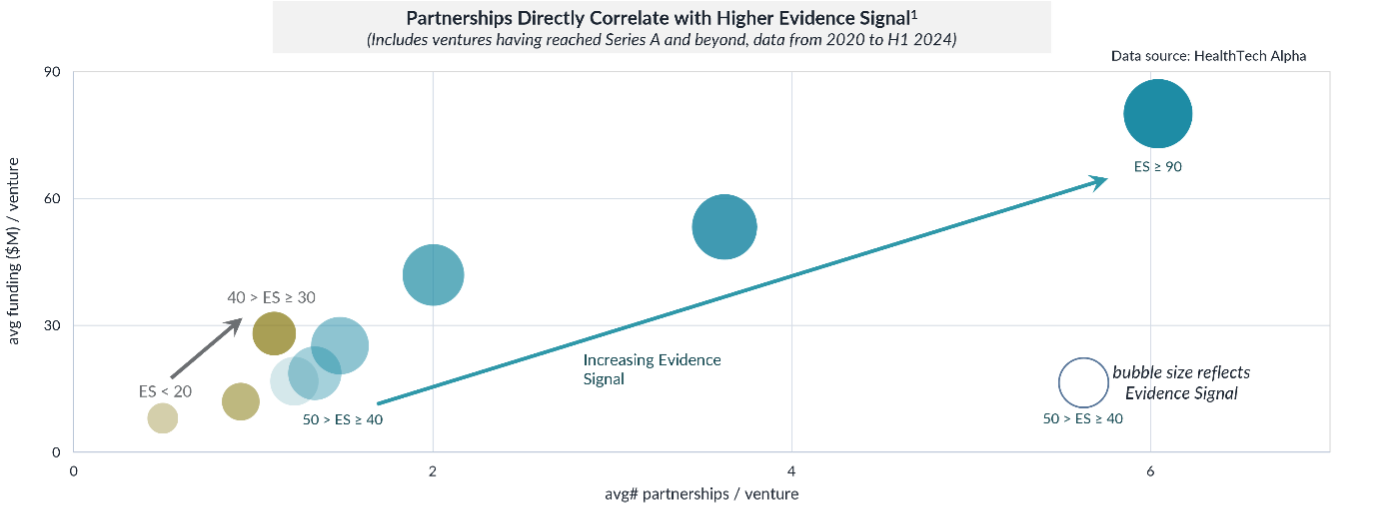
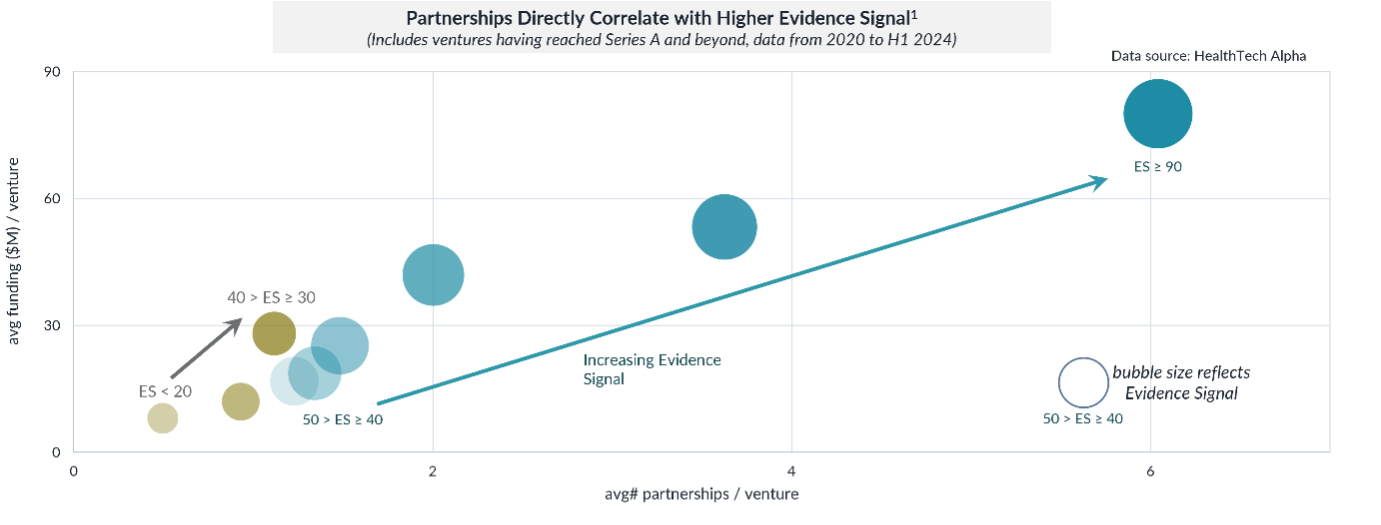
The Growing Importance of Partnerships for Clinical Validation
As digital health expands, partnerships with pharmaceutical companies have become a cornerstone in establishing clinical strength. Since 2020, over 5,100 partnerships involving digital health ventures with proven clinical strength have been announced. These collaborations are pivotal in providing access to expertise, resources, and funding, crucial for conducting clinical trials and achieving regulatory approvals.

Among the industry verticals, healthcare providers (19%) and the pharmaceutical industry (15%) have led the charge in forming these partnerships. This is no surprise, given the importance of evidence of safety and efficacy in healthcare settings. Collaborating with well-established pharmaceutical companies enhances the credibility of digital health ventures and signals to the market that these solutions have undergone rigorous evaluation.
The Role of Clinical Trials, Regulatory Approvals, and Publications
Clinical trials remain the gold standard for demonstrating the efficacy and safety of digital health solutions. According to the data, the number of clinical trials in digital health dipped in 2022 as a result of the Covid-pandemic but recovered in 2023 and has increased, from just 92 trials in 2016 to a staggering 512 in the first half of 2024. This growth reflects the sector’s commitment to validating new technologies and ensuring they meet the highest healthcare standards.
In a similar trend, regulatory approvals reached their highest point in 2021 and have been gradually decreasing since. Approvals from bodies like the FDA or EMA act as a mark of credibility, confirming that solutions adhere to strict safety and efficacy standards. The number of approvals climbed from 269 in 2016 to 942 in 2021, before settling at 456 in 2023, highlighting the growing focus on regulatory compliance.
Publications in peer-reviewed journals further cement the clinical strength of digital health solutions. These publications provide transparency and scientific rigour and allow the broader medical community to scrutinise and validate findings. With nearly 7,800 publications in each of the years from 2021 through 2023, the digital health sector prioritises disseminating validated scientific knowledge.
Areas of Digital Health Needing Proven Clinical Strength
While clinical strength is important across all areas of digital health, certain categories require more rigorous validation due to their direct impact on patient health and safety.
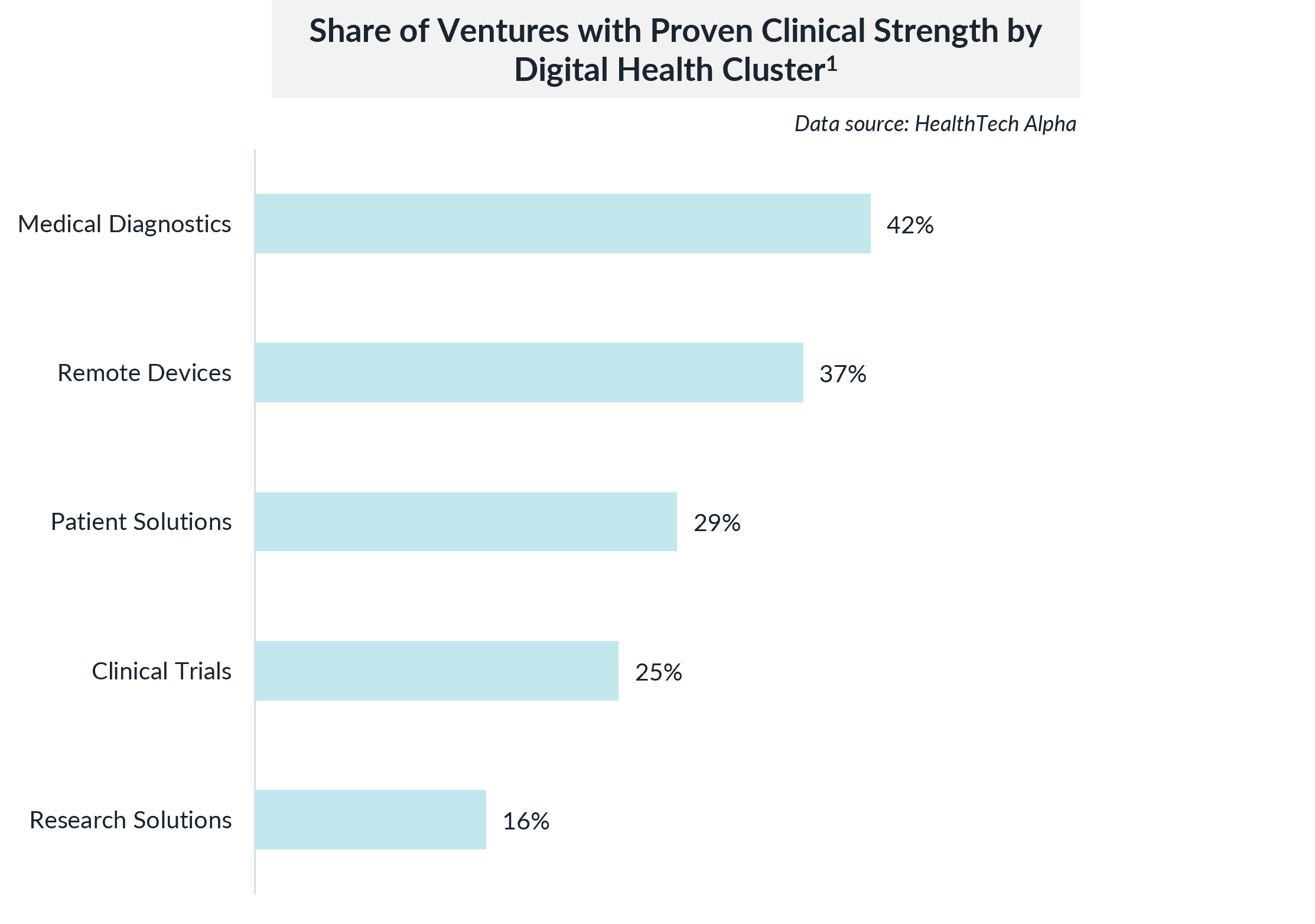
For instance, Remote Monitoring Devices must demonstrate high accuracy and reliability, which is crucial in managing chronic conditions. Currently, 37% of ventures in this cluster have achieved proven clinical strength, reflecting the sector’s commitment to evidence-based innovation. Similarly, 29% of ventures in the Patient Solutions cluster, which includes digital therapeutics and health tracking apps, have demonstrated clinical strength, a critical factor in earning the trust of both healthcare providers and patients.
Evidence-Based Investment Trends
Investors are increasingly focusing their funds on ventures that can generate solid clinical validation in digital health. An analysis of funding trends from 2023 and the first half of 2024 shows that, on average, 40% of ventures with proven clinical strength secure 59% of the total funding in their respective clusters. This illustrates that ventures with validated clinical strength are more likely to attract investment, as they present lower risk and higher potential for successful market integration.

Looking Ahead: The Future of Clinical Evidence in Digital Health
As the digital health landscape continues to evolve, the importance of clinical evidence will only grow. Areas such as remote monitoring, digital therapeutics, and diagnostic tools will need to continue prioritising clinical validation to ensure they deliver tangible health benefits and gain the trust of all stakeholders in the healthcare ecosystem.
Partnerships with health systems, pharmaceutical companies and other ecosystem participants will remain crucial in this journey. These collaborations provide the necessary resources for conducting robust clinical studies, helping to navigate complex regulatory landscapes and achieve market access more efficiently.
Conclusion: Subscribe for More Insights
Clinical strength is the bedrock of trust and credibility in digital health. As we prepare to release our full report at the end of September, we invite you to subscribe to be notified or monitor our research page. This report will delve deeper into the trends, challenges, and opportunities in building clinical strength in digital health, offering valuable insights for healthcare providers, investors, and industry leaders.
Stay ahead of the curve—subscribe now to gain early access to this essential resource.