Unlocking Speed, Safety, and Savings — why digital innovation and global policy alignment are the new catalysts in drug development
TL;DR
- The UK government’s reforms and use of AI have halved clinical trial approval times, signalling a global shift toward faster, smarter research.
- The FDA’s latest digital evidence guidance complements this, marking regulatory momentum on both sides of the Atlantic.
- Despite a 46% decline in global funding for digital clinical trials since 2020, innovation is accelerating — especially in AI-driven design, recruitment, and decentralised trial models.
- Galen Growth data show that 99% of venture funding in this space in 2025 has gone to AI-powered solutions.
- The opportunity: align capital, policy, and partnerships to make clinical trials faster, cheaper, and safer — and bring life-saving therapies to patients sooner.
Digital technology meets regulatory momentum in digital clinical trials
The UK government’s announcement that clinical trial approval times have been cut by half marks a watershed moment in medical innovation. Underpinned by artificial intelligence and regulatory reform, the country’s new streamlined process signals a renewed focus on accelerating drug development while maintaining safety and rigour (UK Medicines and Healthcare Regulatory Agency, 1 Oct. 2025, UK clinical trial approval times twice as fast with AI and reforms).
According to the Department of Health and Social Care, AI tools and digitalised approval pathways introduced in 2024 have reduced average approval timelines to around 30 days — down from 60 — with complex studies seeing even more dramatic improvements. The reforms also simplify ethics approvals and data sharing between the Medicines and Healthcare Products Regulatory Agency (MHRA) and the National Health Service (NHS).
This is not a localised success story. It reflects a broader trend reshaping the global life sciences ecosystem: digital transformation is redefining how clinical research is designed, run, and regulated.
The regulatory picture on both sides of the Atlantic
The UK’s reform echoes similar efforts by the US Food and Drug Administration (FDA), which recently issued new guidance on digital health technologies (DHTs) and AI in clinical investigations. The FDA’s framework expands the use of digital evidence — from wearables and decentralised trial data to real-world data integration — to support submissions and regulatory decision-making.
Together, these moves represent a critical step toward closing the gap between technological innovation and regulatory adoption. For decades, the bottleneck in drug development has been the clinical trial itself — the most expensive, risky, and time-consuming stage of the entire process.
The drug development bottleneck in numbers
As Galen Growth’s 2025 Digital Innovation in Clinical Trials report underscores, clinical trials account for more than half of total R&D costs, with individual trials costing $1 million to $100 million and averaging $15,000–$50,000 per patient. Recruitment delays, data fragmentation, and regulatory complexity remain the leading causes of overrun.
The financial pressure is intensifying. The report highlights that global funding for ventures developing digital tools for clinical trials has declined by 46% in the past five years, falling from $2.4 billion in 2020 to $1.3 billion in 2024, and that the cluster’s share of total Digital Health funding has dropped from 6.9% to 4.8%.
Yet, the data also reveal extraordinary momentum beneath the surface: AI-driven clinical trial ventures now account for 99% of all funding in 2025. Moreover, 89% of new ventures founded since 2023 have embedded AI into their solutions — up from 69% in earlier years.
This is not a contraction. It is consolidation and acceleration — a pivot from hype to impact.
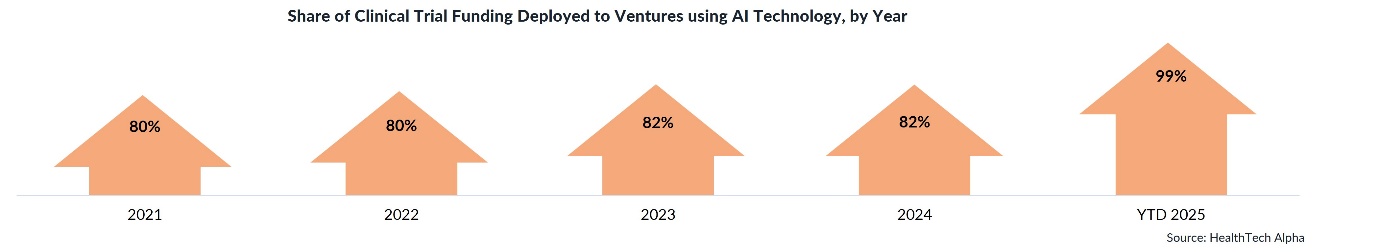
AI is transforming every phase of the trial process
The implications are profound. From trial design to recruitment, monitoring, and data analysis, artificial intelligence is making trials faster, cheaper, and safer.
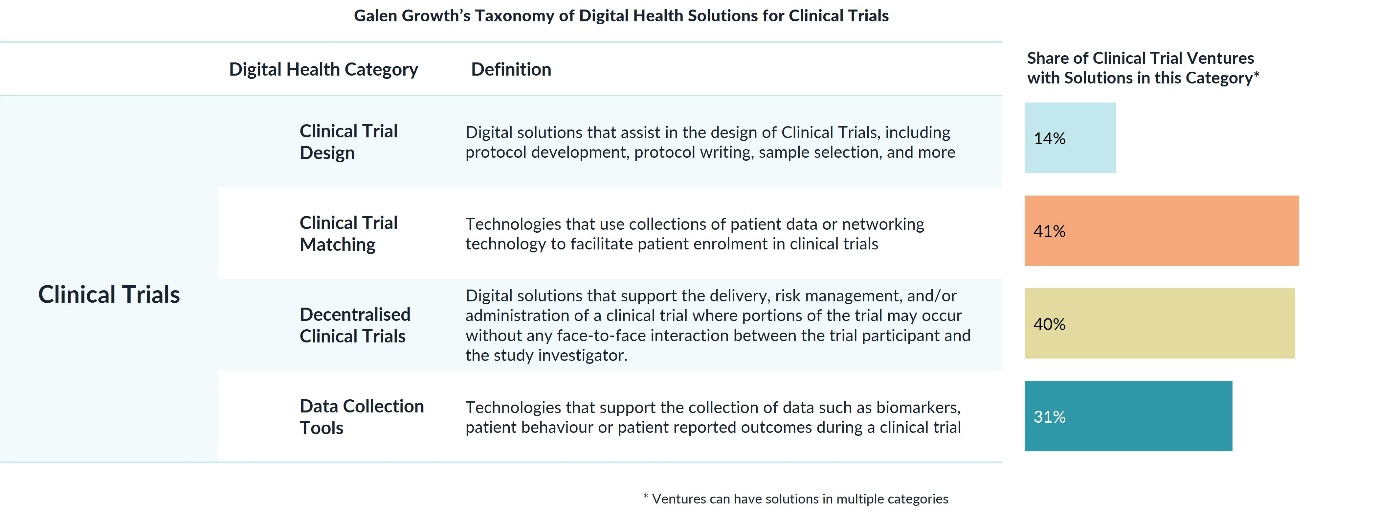
AI-driven design tools can analyse years of prior research, bioinformatics, and regulatory data to optimise protocols before a trial begins. Galen Growth data show that 86% of ventures in clinical trial design now use AI, reducing failures and improving success rates in later phases. Companies such as Pathos — which recently secured a $365 million Series D round — exemplify this new wave of predictive trial optimisation.
Recruitment remains the industry’s Achilles’ heel. AI platforms can now match patients to suitable studies in seconds, drawing on electronic health records, genomic data, and real-world evidence. The report highlights that 71% of ventures in clinical trial matching leverage AI, while the NIH’s “TrialGPT” large language model demonstrates how automation can identify eligible participants faster and more inclusively than traditional manual screening.
3. Decentralised Clinical Trial Platforms
Decentralisation — allowing patients to participate remotely via telehealth, wearables, and home monitoring — is reshaping accessibility. Ventures like the UK’s Lindus Health, which raised $55 million in 2025, are pioneering hybrid models that bring studies directly to patients. These platforms not only reduce costs, administrative tasks, and travel burdens but also improve diversity and retention — key priorities for regulators and sponsors alike.
Real-time data and oversight using AI and blockchain-secured consent are enabling continuous, high-integrity monitoring. This not only ensures patient safety but also enables early corrective action during trials, reducing the risk of costly failure.
Galen Growth’s Opinion: Health systems and regulators are finally catching up
Historically, regulators and health systems lagged behind technology developers. That gap is closing.
The NHS is building open-access trial registries to improve transparency and public trust. Meanwhile, both the MHRA and FDA are adopting risk-based oversight models that allow remote monitoring and adaptive design methodologies.
This alignment between innovators and regulators marks a decisive cultural shift: from viewing digital tools as experimental to recognising them as essential infrastructure.
Still, as the Galen Growth report cautions, “capital remains misaligned.” The technology to transform clinical trials already exists — what’s needed now is scale.
The investment paradox
Despite strong proof points, the sector’s funding gap remains stark. Only 10% of ventures launched in the past five years have successfully raised a Series A round, and many struggle to survive before reaching maturity. Yet, once established, 25% of older ventures have exited, the highest rate across all Digital Health clusters.
| Date | Venture | Acquirer | Primary Category | Country |
| June 2025 | Circuit Clinical | Lightship | Decentralised Clinical Trials | United States |
| March 2025 | Deep 6 AI | Tempus AI | Clinical Trial Matching | United States |
| March 2025 | Mondosano | Clariness | Decentralised Clinical Trials | Germany |
| Jan 2025 | Real Life Sciences | MediSpend | Data Collection Tools | United States |
| Jan 2025 | Syapse | N-Power Medicine | Clinical Trial Matching | United States |
This suggests a ripe but underfunded opportunity. Investors seeking scalable, AI-driven efficiency plays in life sciences should look beyond drug discovery — toward clinical trials, where gains in cost and time efficiency directly translate into accelerated revenue realisation for pharmaceutical sponsors.
Galen Growth Point of View
Galen Growth’s analysis provides a clear message: the clinical trial bottleneck is not a technology problem — it is a coordination problem.
“The technologies to make trials faster, cheaper, more inclusive, and more reliable already exist,” the report concludes. “What’s needed is strategic funding, regulatory alignment, and bold partnerships to scale these innovations across the industry.”
The UK’s accelerated approval reforms are more than bureaucratic efficiency — they symbolise the beginning of a new era in evidence generation. By combining AI, open data, and clear regulatory frameworks, the UK and the US are showing how digital infrastructure can turn the clinical trial bottleneck into a catalyst for faster, fairer innovation.
Yet, progress will only endure if public policy, private investment, and global regulatory cooperation continue to move in concert.
Digital innovation has proven it can deliver the tools. Now, capital and coordination must catch up.
Why this matters: The strategic point of view
For industry leaders, faster, digitally enabled trials mean shorter time-to-market and reduced cost-per-asset — essential in an era of squeezed margins and complex biologics.
For investors, the combination of AI adoption and regulatory support creates a rare window to back scalable infrastructure that can serve both pharma and health systems globally.
For startups, the message is one of opportunity and urgency. As venture capital shifts back toward efficiency and tangible ROI, proven digital enablers in trial design, patient recruitment, and decentralisation will command outsized valuations.
In short, the industry’s digital transformation no longer hinges on discovery. It depends on delivery.
About Galen Growth and HealthTech Alpha
Galen Growth is the global leader in Digital Health intelligence, trusted by pharma, med-tech, investors, insurers, and health systems to navigate and benchmark innovation.
Its flagship platform, HealthTech Alpha, is the most comprehensive data and analytics engine for digital technology innovation in healthcare, covering over 50,000 ventures and more than one billion data points.
From deal flow and partnerships to evidence validation and AI-powered analytics, HealthTech Alpha enables clients to make faster, smarter, and data-driven decisions in an increasingly complex ecosystem.
In 2025, as the industry moves from hype to health impact, Galen Growth continues to redefine Digital Health actionable intelligence — delivering clarity where others deliver noise.